A team of researchers at McMaster University (Canada) recently confirmed previous observations that the composition and amount of mycobacteria (gut bacteria) in the large intestine changes with age. This process led to intestinal inflammation in experimental mice, which ultimately shortened the lifespan of the animals.
In a paper co-authored by the researchers (published in the journal Cell Host & Microbe), they show that interventions that modify the microbiological composition of the gut (the so-called gut microbiome), including microbiome transplantation, probiotics and prebiotics, can rebalance the gut microbiota, which can restore the healthy functioning of the gut and thus prevent diseases of this origin in old age.
As we age, inflammatory processes take place in our intestinal tract, in most cases without any particular symptoms or signs, so the host of the process is not really aware of it. It is also known that people with higher levels of inflammatory markers in their blood are more likely to be less healthy, die earlier, be less active and suffer from more chronic inflammatory diseases (dementia, cardiovascular disease).
Individuals with inflammatory conditions need hospital treatment more often and require more care. Despite this, until now we did not really know what was behind the increased inflammation in older people.
A newly published Canadian study is the first to clearly describe a causal link between variation in the gut microbiome and inflammation levels in experimental animals (mice).
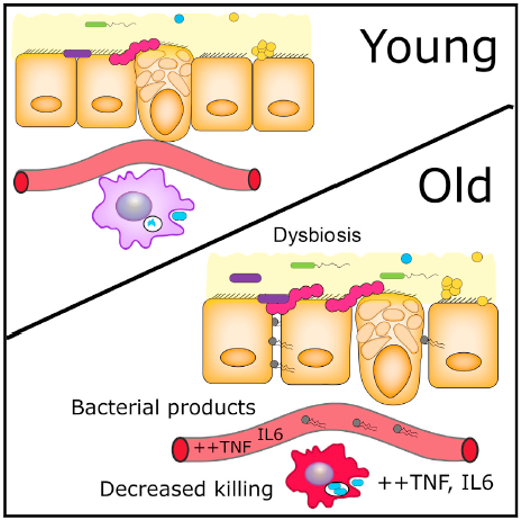
The discovery is that a long-term negative change in the composition of the gut microbiome increases the permeability of the gut – including to pathogenic microorganisms – which, when they enter the bloodstream, trigger inflammatory processes, impairing the immune defence and ultimately reducing the life expectancy of the affected individual.
The function of macrophage cells, which play a central role in triggering immune defence, is clearly impaired in a constant inflammatory environment, with cells less able to protect the host organism effectively. It is hypothesised that the persistence of an imbalance in the gut flora (‘dysbiosis’) may lead to the development of a persistent inflammatory environment – and that counteracting this may greatly improve the body’s defences and, based on experimental results, is likely to improve the individual’s life expectancy. The best antidote to dysbiosis is a balanced healthy diet, adequate physical activity and, if the bacterial balance has already been disrupted, restoration of the balance by transplanting a suspension of healthy individuals’ colonic flora and/or the use of pre- and probiotic preparations. The latter theory is currently the subject of much current scientific research.
Reference:
Thevaranjan et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe
https://www.cell.com/cell-host-microbe/abstract/S1931-3128(17)30112-9


